Search
CLOSEStereoselectivity of reactions catalyzed by transition metals
Dr. Emmanuel Simandiras
Group leader
Dr. Emmanuel SimandirasResearch Director
Theoretical Inorganic and Organometallic Chemistry
- Transition metal complexes with interesting electronic properties
- Stereoselectivity of reactions catalyzed by transition metals
- Quadruple metal-metal bonds and their protonation
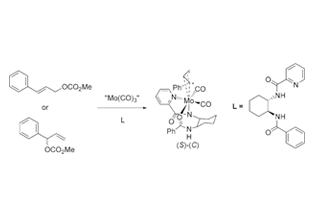
A number of organic reactions catalyzed by transition metal complexes produce products that are richer in a certain stereoisomer, leading to stereoselective procedures. The allylic alkylation catalyzed by Mo complexes was pioneered by Trost at Stanford University in 1998. We performed extended mechanistic studies of these reactions and using highly accurate computations of a great number of transition states, we managed to explain the streoselectivity in the process. Structural effects were explained and the role of hydrogen bonding elucidated.
Key Publications
Group leader
Dr. Emmanuel SimandirasResearch Director
Theoretical Inorganic and Organometallic Chemistry
- Transition metal complexes with interesting electronic properties
- Stereoselectivity of reactions catalyzed by transition metals
- Quadruple metal-metal bonds and their protonation