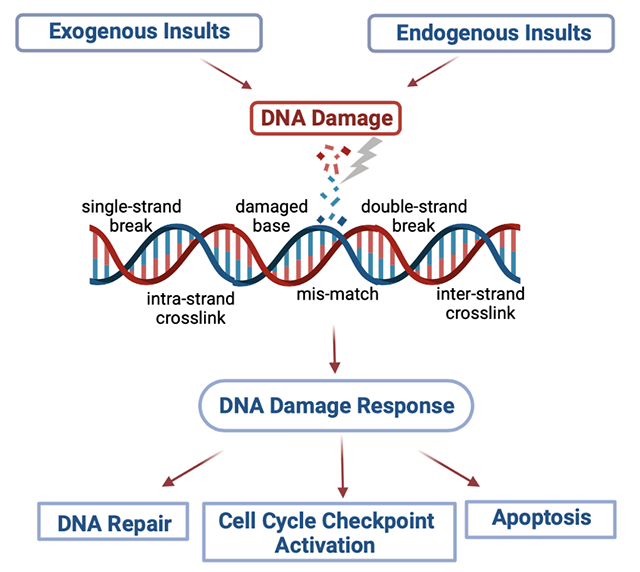
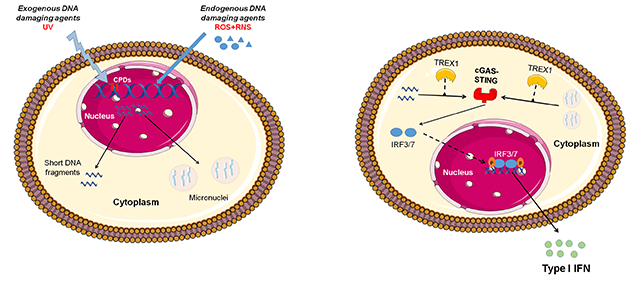
The human genome is constantly subjected to endogenous and exogenous sources of damage. Protection against these genotoxic insults is secured by the network of DDR pathways triggered by the detection of DNA lesions. The subsequent step is the initiation of a signal transduction cascade including molecules that activate genome-protection pathways, such as DNA repair, cell cycle control, apoptosis, transcription and chromatin remodeling. Failure to repair DNA damage can result in a variety of genomic alterations, such as point mutations, chromosomal translocations and gain or loss of chromosomal segments or entire chromosomes. Under certain conditions, these genomic aberrations induce changes in cellular physiology that drive disease initiation and progression. In this project, using established cell lines and primary biological samples from cancer patients (multiple myeloma, head and neck cancer, lung cancer) and autoimmune diseases (systemic lupus erythematosus, rheumatoid arthritis, systemic sclerosis, Behçet's disease, antiphospholipid syndrome) at different stages of the disease, we test the hypothesis that the deregulated DDR network plays a crucial role in the onset and progression of cancer and autoimmunity.
Major outcomes
- Systemic autoimmune diseases: The results presented in this study suggest that the deregulated DDR network plays a crucial role in the pathogenesis and progression of systemic autoimmune diseases.
https://doi.org/10.3390/ijms21010055 - Systemic sclerosis (SSc): We found that SSc patients displayed increased endogenous DNA damage and oxidative stress, defective DSB repair but not NER capacity, and deregulated expression of DDR-associated genes.
https://doi.org/10.3389/fimmu.2020.582401 - Rheumatoid arthritis (RA): We found that deregulated chromatin organization, deficient DNA repair capacity and augmented formation of DNA damage, which are reversible after treatment, contribute to the accumulation of endogenous DNA damage in RA.
https://doi.org/10.1016/j.clim.2019.03.009 - RNA editing: We found that ADAR1p150-mediated A-to-I RNA editing is critically involved in type I IFN responses highlighting the importance of proinflammatory gene regulation at the post-transcriptional level in systemic autoimmunity.
https://doi.org/10.1016/j.jaut.2021.102755 - Iron deposition and DDR activation in Systemic Sclerosis: In this study, we formulated the hypothesis that hemorrhagic tissue deposition of iron, due to microvasculopathy-related extravasation of erythrocytes, may be of pathogenetic importance in fibrosis in SSc, thus comprising an additional therapeutic target.
https://doi.org/10.3390/life12030430 - Genomic instability/carcinogenesis: The current study broadens our understanding of how chronic p53-independent p21WAF1/Cip1 expression, seen in a sizeable fraction of advanced human tumors, impacts the global DNA repair landscape and undermines genomic stability.
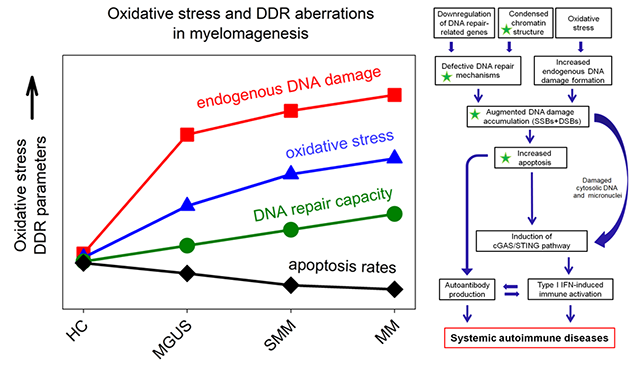
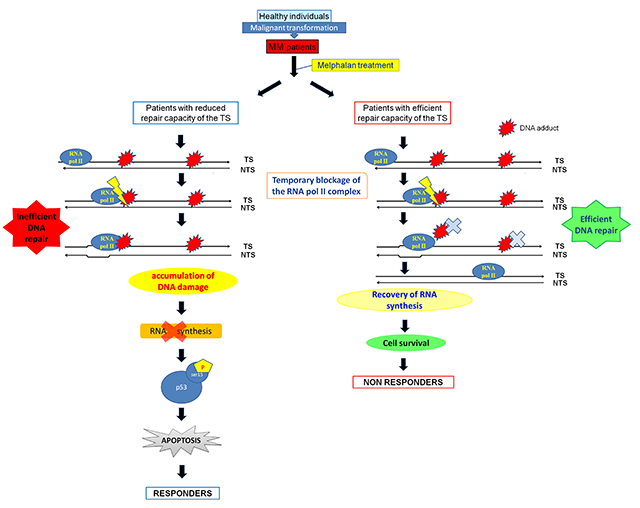
https://doi.org/10.1186/s13059-018-1401-9 - DDR in multiple myeloma: In this study, we tested the hypothesis that epigenetic alterations and changes in DDR signals are implicated in the transformation process of myelomagenesis. Indeed, we found that in bone marrow plasma cells from patients with MGUS, SMM and MM, significant progressive changes occur in chromatin structure, transcriptional activity and DDR signals during the transformation process of myelomagenesis. Interestingly, these changes were also present in PBMCs from the same patients and strongly correlated with those observed in corresponding bone marrow plasma cells.
https://doi.org/10.1038/leu.2013.284