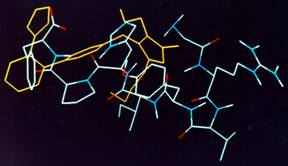
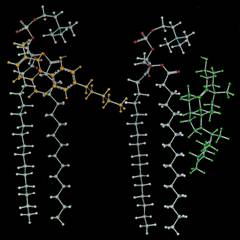
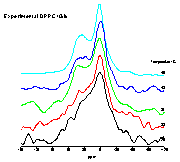
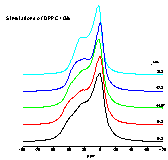
|
Greek |
|
|||||||||||
|
|
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||
Last Update: 05.07.2006
| ę National Hellenic Research Foundation (NHRF), 48 Vassileos Constantinou Ave., 11635 Athens, Greece, Tel. +302107273700, Fax. +302107246618 |